
The Periodic Table of Oxidation States Oxidation state, Teaching
The oxidation number of a monatomic ion (by itself or as part of an ionic compound) is equal to its charge. Alkali metals - elements in the first column of the periodic table - will always have an oxidation number of +1; Alkali metals (column 2) are almost always +2

Oxidation Number (State) Definition, Rules, How to Find, and Examples
These have oxidation numbers of +2 & +3 respectively. With a chlorine ion (a chlorine atom that has gained one electron, Cl -), the oxidation number would be -1. Oxidation state 0 occurs for all elements - it is simply the element in its elemental form. As the table shows, the presence of the other oxidation states varies, but follows.

The Periodic Table of Oxidation States Compound Interest
Using a list of simple rules you'll learn how to find the oxidation numbers for elements and compounds. For each rule there are examples and practice calcul.
The Periodic Table of Oxidation States Compound Interest
This. Oxidation States table gives the Oxidation States of all the elements of periodic table. Click on 'Element Atomic Number', 'Element Symbol', 'Element Name' and 'Element Oxidation States' headers to sort.
Aluminum Aluminum Oxidation Number
Interactive periodic table showing names, electrons, and oxidation states. Visualize trends, 3D orbitals, isotopes, and mix compounds.. 3D orbitals, isotopes, and mix compounds. Fully descriptive writeups. Periodic Table of Elements. Properties Electrons Isotopes Compounds. or buying a poster or wallet card, order number. 1H Hydrogen 1..
PPT Elements and Periodic Table PowerPoint Presentation, free
The oxidation number of any free element is 0. The atoms in Na, O 2, N 2, Pb, He, H 2, Ne, Zn, for example, have oxidation numbers of 0.. The oxidation number of a monatomic ion is equal to the charge on the ion. For example, the oxidation number of chlorine in the Cl-ion is -1. The oxidation number of bromine in the Br-ion is -1. The oxidation number of sodium in the Na + ion is +1.

The Periodic Table of Oxidation States Compound Interest
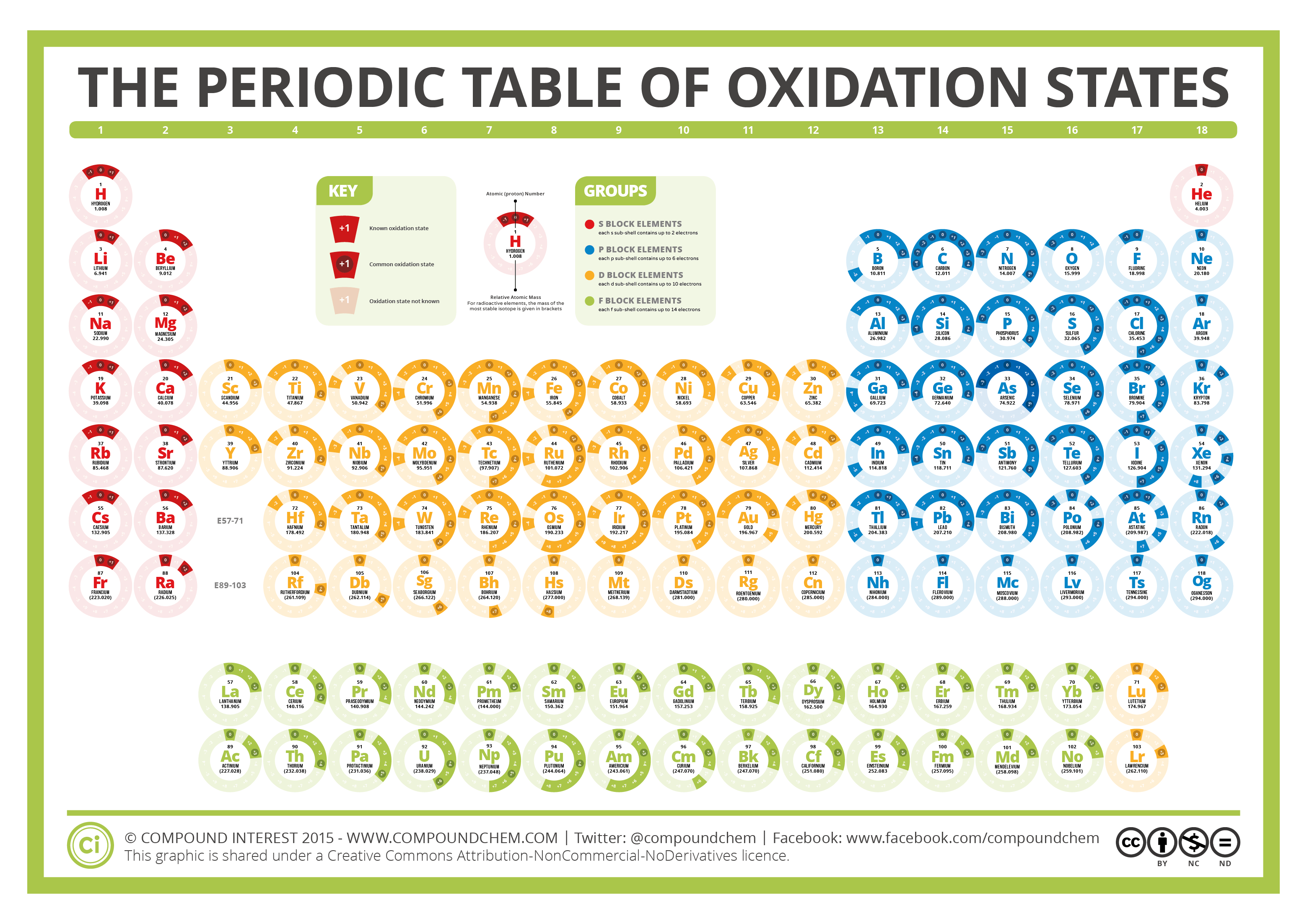
EDIT: This table has been updated to include corrections in the atomic masses of iron and gallium. The corrected table can be found here. This color periodic table contains the number, symbol, name, atomic mass and oxidation states of each element. The most common oxidation states are in bold text and predicted or unconfirmed states are in italics.

The Periodic Table of Oxidation States Compound Interest
This number is defined as the formal charge on the atom if all bonds were assumed to be fully ionic. Knowing the oxidation state is very useful for example when balancing reduction-oxidation (redox) reactions. Figure 1: Oxidation states in the Periodic Table. Referred from: Article. Oxidation numbers for atoms, ions and compounds.

Nomenclature
Oxidation Numbers. An oxidation number is a positive or negative number that is assigned to an atom to indicate its degree of oxidation or reduction.The term oxidation state is often used interchangeably with oxidation number. A partial electron transfer is a shift in the electron density near an atom as a result of a change in the other atoms to which it is covalently bonded.
The Nameinator Finding Oxidation Numbers
The oxidation number for O is 2- in most compounds. In this video, 2- is written above O. Another way to think about it is 1- being written above O2. Also check out the video about unusual oxygen oxidation states.. Now, let's go to the other side of the periodic table to Group 7, the halogens. The halogens right over here, they're quite.

Downloadable Periodic Table Oxidation States
Oxidation number of an atom when an element has combined with the same element. When an element has combined with same kind element, it's oxidation number becomes 0. Ex: oxidation number of Bromine in Br 2 molecule is 0. Table: oxidation numbers of first 20 elements in the periodic table according to the atomic number

Downloadable Periodic Table Oxidation States
9. The sum of the oxidation numbers in a neutral compound is zero. H 2 O: 2(+1) + (-2) = 0. 10. The sum of the oxidation numbers in a polyatomic ion is equal to the charge on the ion. The oxidation number of the sulfur atom in the SO 4 2-ion must be +6, for example, because the sum of the oxidation numbers of the atoms in this ion must equal -2.
/PeriodicTableOxidation-BW-56a12da83df78cf772682bfe.png)
Periodic Table With Groups Numbers Periodic Table Timeline
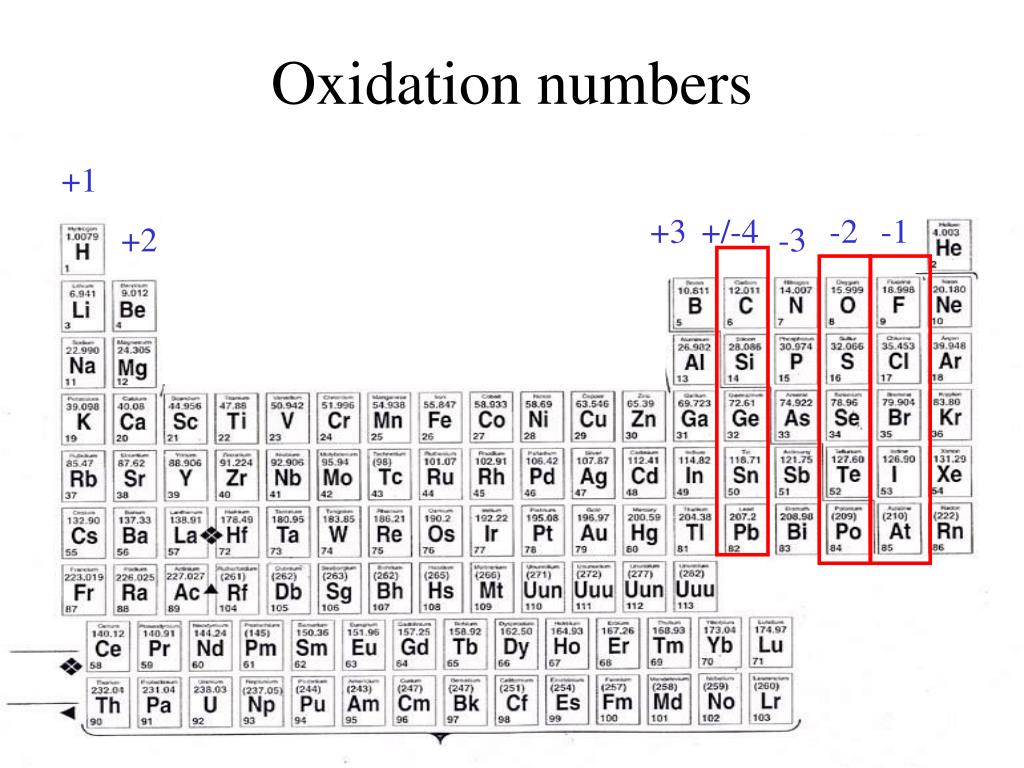
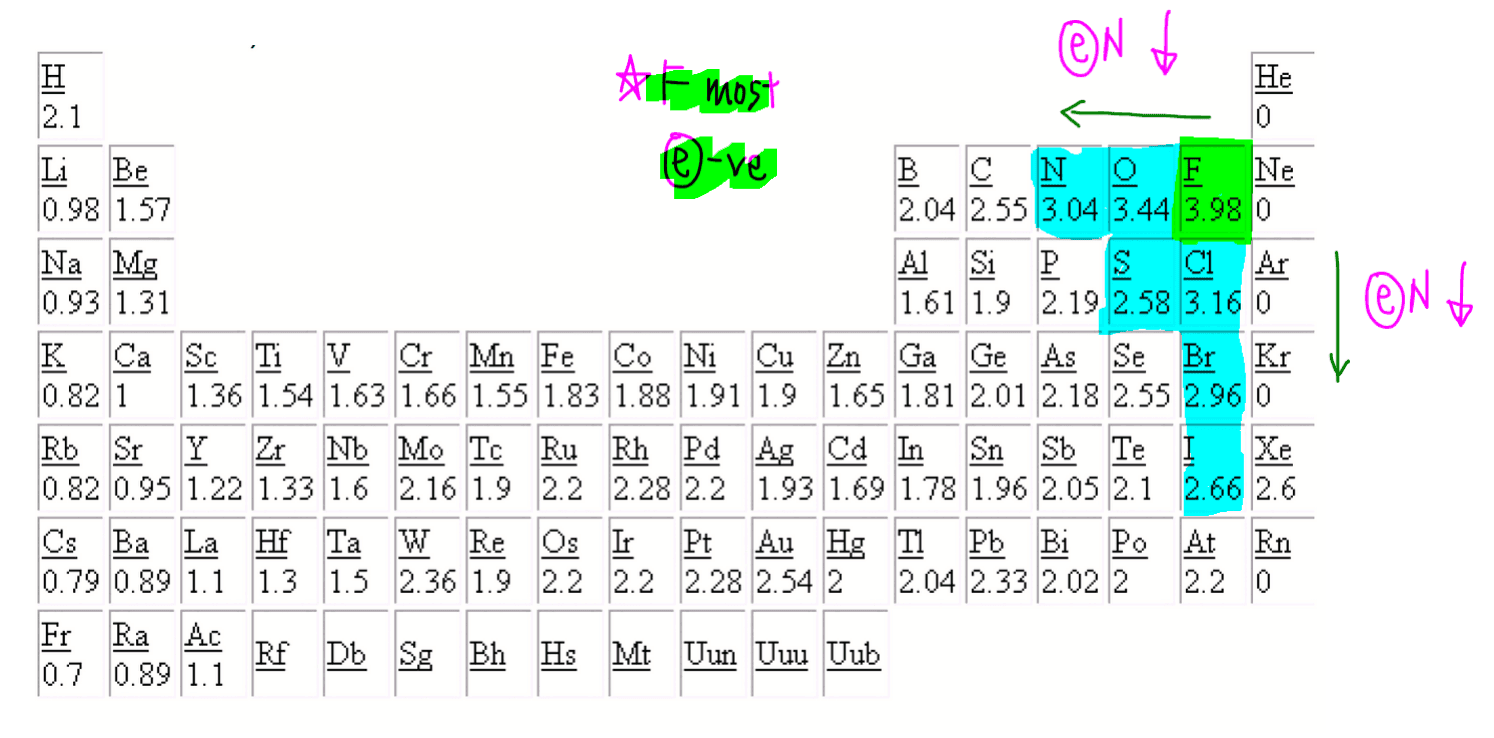
The positive oxidation state is the total number of electrons removed from the elemental state. It is possible to remove a fifth electron to form another the \(\ce{VO_2^{+}}\) ion with the vanadium in a +5 oxidation state.. Remember that electronegativity is greatest at the top-right of the periodic table and decreases toward the bottom-left.

The Periodic Table of Oxidation States Oxidation state, Teaching
This periodic table contains the atomic number, element symbol, element name, atomic weights and oxidation numbers. Todd Helmenstine. This periodic table contains the oxidation numbers of the elements. Bold numbers represent the more common oxidation states. Values in italics represent theoretical or unconfirmed oxidation numbers. This table.
Periodic Table Of Oxidation States
Enter the formula of a chemical compound to find the oxidation number of each element. A net ionic charge can be specified at the end of the compound between { and }. For example: ZnCl4{2-} or NH2NH3{+}. Enter just an element symbol to show the common and uncommon oxidation states of the element.
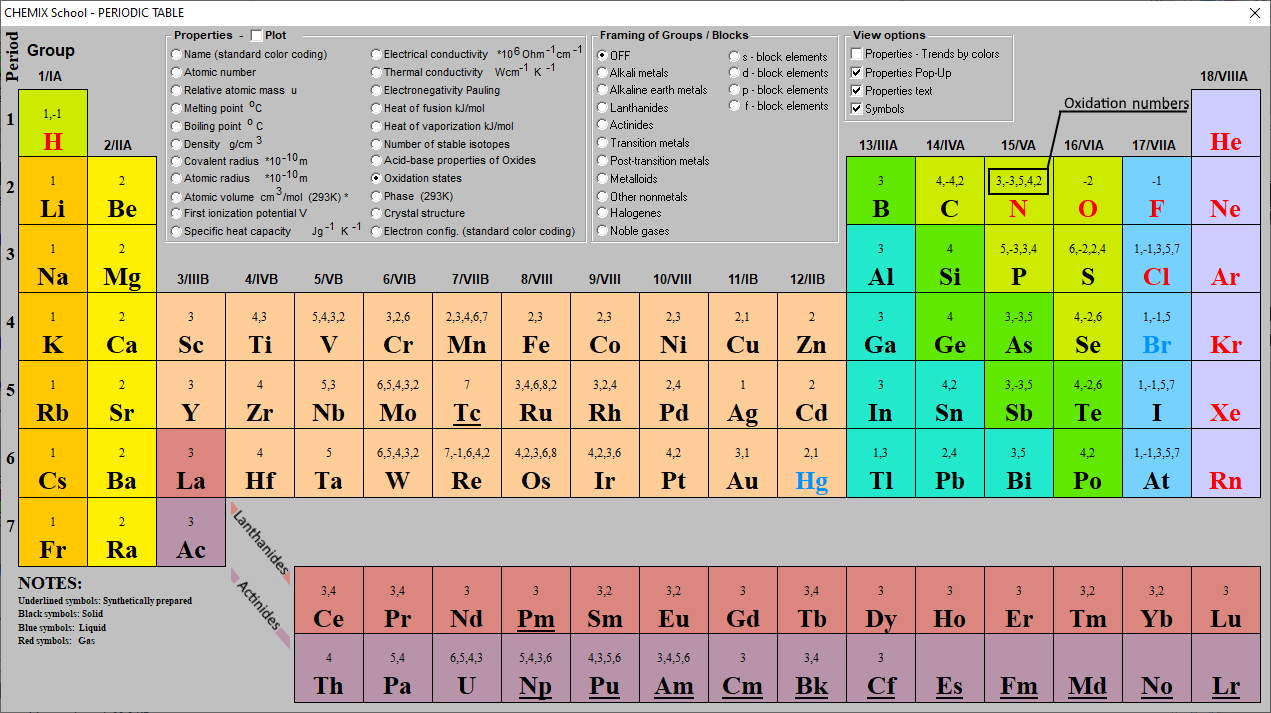
Oxidation Numbers Periodic Table Elements
Oxidation numbers; Monoatomic ions oxidation numbers; Polyatomic ions oxidation numbers; Periodic table with oxidation numbers; Steps to writing chemical formulas; Molecular and Empirical Formulas; Molecular mass and formula mass; Types of chemical compounds; Binary compounds; Ternary compounds